Peptide Reconstitution 101: Understanding How Peptides Mix for Research Use
Many research peptides come as a white, freeze-dried powder called a lyophilized puck.Before researchers can begin studying a material, this powder is typically combined with acompatible laboratory solution — a
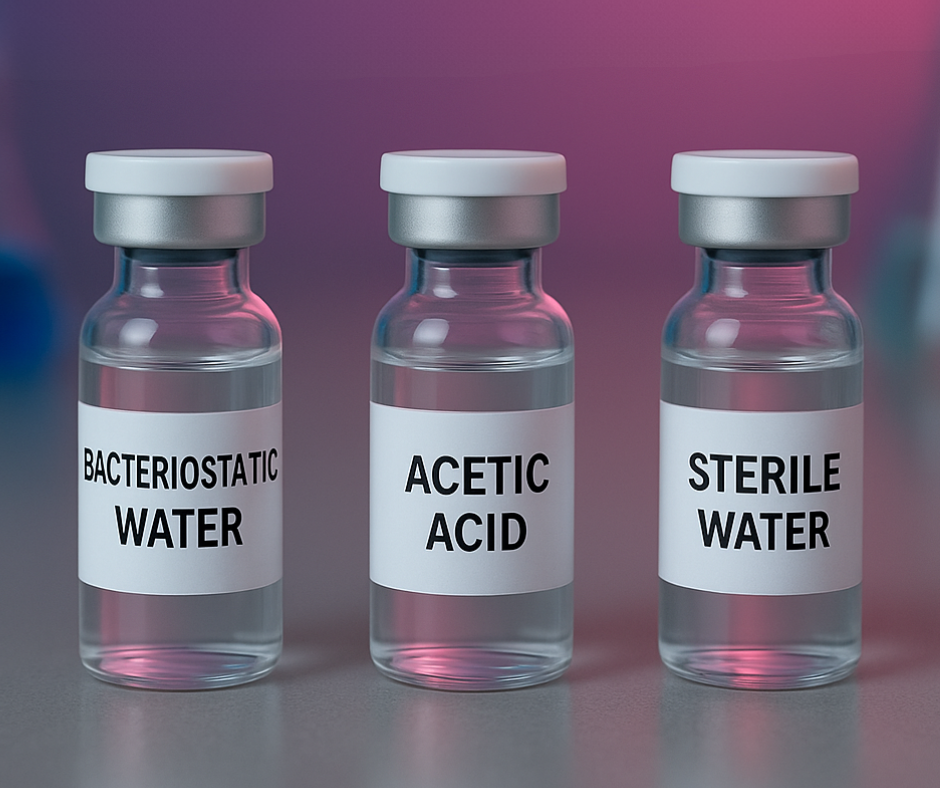
Table of Contents
Many research peptides come as a white, freeze-dried powder called a lyophilized puck.
Before researchers can begin studying a material, this powder is typically combined with a
compatible laboratory solution — a process known as reconstitution.
This guide explains the science behind reconstitution so researchers can better understand what
they’re observing in the lab.
Reminder:
These compounds are sold strictly for laboratory research.
We supply the materials — each researcher is responsible for performing their own study, their
own procedures, and their own due diligence.
This article does not provide instructions, measurements, or usage guidance.
Why Peptides Come Lyophilized
Peptides are sensitive molecules. Lyophilization (freeze-drying):
- protects their structure
- increases stability
- prevents premature breakdown
- improves long-term storage
- makes transportation safer
The white “puck” is simply the peptide in its most stable and preserved form.
Why Reconstitution Behaves Differently From Peptide to Peptide
Not all peptides reconstitute the same way — and this is completely normal. Several scientific
factors influence how a lyophilized puck interacts with solution:
1. Amino Acid Sequence & Charge
A peptide’s sequence determines:
- how hydrophilic or hydrophobic it is
- how easily it interacts with water
- whether it prefers slightly acidic or basic environments
This is why one peptide dissolves immediately while another takes more time.
2. Solubility Profile
Every peptide has a unique solubility pattern.
Normal behaviors include:
- instantly dissolving
- dissolving slowly
- forming a temporary foam
- creating micro-bubbles
- lightly sticking to the vial walls
None of these indicate anything about purity or quality.
3. Lyophilization Pattern
Freeze-dried pucks may appear:
- cracked
- shrunken
- fluffy
- centered or off-center
- adhered to the side
This affects how the solvent initially hits the surface, not the peptide’s integrity.
4. Temperature & Handling
Cool temperatures slow mixing.
Gentle swirling encourages incorporation.
Aggressive shaking is avoided in research settings.
Common Observations (All Normal in a Lab
Setting)
- Powder stuck to the glass: static from freeze-drying
- Instant dissolution: strong water affinity
- Slow dissolution: typical for certain sequences
- Light foam: air introduction
- Bubbles: early mixing behavior
These variations are expected in peptide study work.
Solvents Used During Laboratory
Reconstitution
Different peptides interact differently with various solutions.
Researchers choose solvents based on the peptide’s chemistry, not one universal rule.
Common research-grade solutions include:
Bacteriostatic Water (BAC Water)
Contains a small amount of preservative.
Often chosen when researchers plan extended study after reconstitution.
Sterile Water
Contains no preservative.
Often selected for short-term or immediate research work.
Acetic Acid (AA)
A very small percentage of acetic acid can help dissolve certain peptides that prefer a slightly
acidic environment.
Some researchers also choose a BAC/AA mixture when working with peptides that are sensitive
to pH or slow to incorporate into pure water alone.
This is entirely dependent on the peptide’s structure and the researcher’s study design.
Peptides With More Delicate Reconstitution Behavior
While many peptides incorporate smoothly into compatible laboratory solvents, some are known
to have more sensitive solubility profiles based on their amino-acid structure, pH preferences,
and the way they interact with water or slightly acidic environments.
This is completely normal — different peptides behave differently during reconstitution due to
basic chemistry, not quality issues.
Because each peptide has its own characteristics, researchers often perform additional due
diligence to understand how their specific compound behaves in solution.
We cover this topic in more detail in our companion article:
“Peptides With Unique Reconstitution Characteristics — A Simple Chemistry Guide.”
(This breakdown explains why certain peptides dissolve more slowly, prefer slightly acidic
conditions, or behave differently depending on the solvent — all from a chemistry perspective.)
Storage: Before & After Reconstitution
Before Mixing
Lyophilized peptides prefer cool, dry, dark storage for optimal stability.
After Mixing
After reconstitution, stability timelines change, and researchers commonly store prepared materials under refrigerated conditions during study periods.
Final Thoughts
Reconstitution varies naturally based on:
- peptide structure
- solubility
- pH sensitivity
- lyophilization pattern
- chosen solvent
Foaming, slow mixing, puck variations, or sticking to glass are normal laboratory
observations, not flaws.
Peptide Basix provides research-grade materials and educational resources — researchers are
responsible for understanding the properties of each peptide and conducting their work
accordingly.
If you ever have questions about peptide appearance, solubility differences, or batch
documentation, we’re here to help support your research journey.
